Hydrophobia
For the fear of water, see Aquaphobia.
| Look up hydrophobia in Wiktionary, the free dictionary. |
Hydrophobia or hydrophobe may refer to:
Science and medicine[edit]
- Hydrophobe, a term used in chemistry to describe chemical "aversions" of a molecule, or part of a molecule, to water
- Hydrophobia, rabies, the historic name for rabies, especially a set of symptoms of the later stages of an infection, in which the victim has difficulty swallowing, shows panic when presented with liquids to drink, and cannot quench its/their thirst
Art, entertainment, and media[edit]
- Hydrophobia, a video game developed by Dark Energy Digital
- Hydrophobia, a Hungarian disk magazine (1996–1997)
Hydrophobe
For other uses, see Hydrophobia (disambiguation).
In chemistry, hydrophobicity is the physical property of a molecule (known as a hydrophobe) that is seemingly repelled from a mass ofwater.[1] (Strictly speaking, there is no repulsive force involved; it is an absence of attraction.)
Hydrophobic molecules tend to be non-polar and, thus, prefer other neutral molecules and non-polar solvents. Hydrophobic molecules in water often cluster together, forming micelles. Water on hydrophobic surfaces will exhibit a high contact angle.
Examples of hydrophobic molecules include the alkanes, oils, fats, and greasy substances in general. Hydrophobic materials are used for oil removal from water, the management of oil spills, and chemical separation processes to remove non-polar substances from polar compounds.[2]
Hydrophobic is often used interchangeably with lipophilic, "fat-loving." However, the two terms are not synonymous. While hydrophobic substances are usually lipophilic, there are exceptions—such as the silicones and fluorocarbons.
The term hydrophobe comes from the Ancient Greek ὑδρόφοβος, "having a horror of water", constructed from ὕδωρ, "water", and φόβος, "fear".[3]
Contents
[hide]Chemical background[edit]
Main article: Hydrophobic force
The hydrophobic interaction is mostly an entropic effect originating from the disruption of the highly dynamic hydrogen bonds between molecules of liquid water by the nonpolar solute forming a clathrate-like structure around the non-polar molecules. This structure formed is more highly ordered than free water molecules due to the water molecules arranging themselves to interact as much as possible with themselves, and thus results in a higher entropic state which causes non-polar molecules to clump together to reduce the surface area exposed to water and decrease the entropy of the system.[4][5] Thus, the two immiscible phases (hydrophilic vs. hydrophobic) will change so that their corresponding interfacial area will be minimal. This effect can be visualized in the phenomenon called phase separation.
Superhydrophobicity[edit]
Main article: Superhydrophobe
Superhydrophobic surfaces, such as the leaves of the lotus plant, are those that are extremely difficult to wet. The contact angles of a water droplet exceeds 150° and the roll-off angle is less than 10°.[6] This is referred to as the Lotus effect, and is primarily a physical property related to interfacial tension, rather than a chemical property.
Theory[edit]
In 1805, Thomas Young defined the contact angle θ by analyzing the forces acting on a fluid droplet resting on a solid surface surrounded by a gas.[7]
where
 = Interfacial tension between the solid and gas
= Interfacial tension between the solid and gas = Interfacial tension between the solid and liquid
= Interfacial tension between the solid and liquid = Interfacial tension between the liquid and gas
= Interfacial tension between the liquid and gas
θ can be measured using a contact angle goniometer.
Wenzel determined that when the liquid is in intimate contact with a microstructured surface, θ will change to θW*
where r is the ratio of the actual area to the projected area.[8] Wenzel's equation shows that microstructuring a surface amplifies the natural tendency of the surface. A hydrophobic surface (one that has an original contact angle greater than 90°) becomes more hydrophobic when microstructured – its new contact angle becomes greater than the original. However, a hydrophilic surface (one that has an original contact angle less than 90°) becomes more hydrophilic when microstructured – its new contact angle becomes less than the original.[9] Cassie and Baxter found that if the liquid is suspended on the tops of microstructures, θ will change to θCB*:
where φ is the area fraction of the solid that touches the liquid.[10] Liquid in the Cassie–Baxter state is more mobile than in the Wenzel state.
We can predict whether the Wenzel or Cassie–Baxter state should exist by calculating the new contact angle with both equations. By a minimization of free energy argument, the relation that predicted the smaller new contact angle is the state most likely to exist. Stated in mathematical terms, for the Cassie–Baxter state to exist, the following inequality must be true.[11]
A recent alternative criterion for the Cassie–Baxter state asserts that the Cassie–Baxter state exists when the following 2 criteria are met: 1) Contact line forces overcome body forces of unsupported droplet weight and 2) The microstructures are tall enough to prevent the liquid that bridges microstructures from touching the base of the microstructures.[12]
A new criterion for the switch between Wenzel and Cassie-Baxter states has been developed recently based on surface roughness and surface energy.[13] The criterion focuses on the air-trapping capability under liquid droplets on rough surfaces, which could tell whether Wenzel's model or Cassie-Baxter's model should be used for certain combination of surface roughness and energy.
Contact angle is a measure of static hydrophobicity, and contact angle hysteresis and slide angle are dynamic measures. Contact angle hysteresis is a phenomenon that characterizes surface heterogeneity.[14] When a pipette injects a liquid onto a solid, the liquid will form some contact angle. As the pipette injects more liquid, the droplet will increase in volume, the contact angle will increase, but its three-phase boundary will remain stationary until it suddenly advances outward. The contact angle the droplet had immediately before advancing outward is termed the advancing contact angle. The receding contact angle is now measured by pumping the liquid back out of the droplet. The droplet will decrease in volume, the contact angle will decrease, but its three-phase boundary will remain stationary until it suddenly recedes inward. The contact angle the droplet had immediately before receding inward is termed the receding contact angle. The difference between advancing and receding contact angles is termed contact angle hysteresis and can be used to characterize surface heterogeneity, roughness, and mobility.[how?] Surfaces that are not homogeneous will have domains that impede motion of the contact line.The slide angle is another dynamic measure of hydrophobicity and is measured by depositing a droplet on a surface and tilting the surface until the droplet begins to slide. In general, liquids in the Cassie–Baxter state exhibit lower slide angles and contact angle hysteresis than those in the Wenzel state.
Research and development[edit]
Dettre and Johnson discovered in 1964 that the superhydrophobic, Lotus effect phenomenon was related to rough hydrophobic surfaces, and they developed a theoretical model based on experiments with glass beads coated with paraffin or TFE telomer. The self-cleaning property of superhydrophobic micro-nanostructured surfaces was reported in 1977.[15] Perfluoroalkyl, perfluoropolyether and RF plasma formed superhydrophobic materials were developed, used for electrowetting and commercialized for bio-medical applications between 1986 and 1995.[16][17][18][19] Other technology and applications have emerged since the mid 1990s.[20] A durable superhydrophobic hierarchical composition, applied in one or two steps, was disclosed in 2002 comprising nano-sized particles ≤ 100 nanometers overlaying a surface having micrometer-sized features or particles ≤ 100 micrometers. The larger particles were observed to protect the smaller particles from mechanical abrasion.[21]
In recent research, superhydrophobicity has been reported by allowing alkylketene dimer (AKD) to solidify into a nanostructured fractal surface.[22] Many papers have since presented fabrication methods for producing superhydrophobic surfaces including particle deposition,[23] sol-gel techniques,[24] plasma treatments,[25] vapor deposition,[23] and casting techniques.[26] Current opportunity for research impact lies mainly in fundamental research and practical manufacturing.[27] Debates have recently emerged concerning the applicability of the Wenzel and Cassie–Baxter models. In an experiment designed to challenge the surface energy perspective of the Wenzel and Cassie–Baxter model and promote a contact line perspective, water drops were placed on a smooth hydrophobic spot in a rough hydrophobic field, a rough hydrophobic spot in a smooth hydrophobic field, and a hydrophilic spot in a hydrophobic field.[28]Experiments showed that the surface chemistry and geometry at the contact line affected the contact angle and contact angle hysteresis, but the surface area inside the contact line had no effect. An argument that increased jaggedness in the contact line enhances droplet mobility has also been proposed.[29]
Many very hydrophobic materials found in nature rely on Cassie's law and are biphasic on the submicrometer level with one component air. The Lotus effect is based on this principle. Inspired by it, a lot of functional superhydrophobic surfaces were prepared.[30]
An example of a biomimetic superhydrophobic material in nanotechnology is nanopin film. In one study, a vanadium pentoxide surface that can switch reversibly between superhydrophobicity and superhydrophilicity under the influence of UV radiation is presented.[31] According to the study, any surface can be modified to this effect by application of a suspension of rose-like V2O5 particles, for instance with an inkjet printer. Once again hydrophobicity is induced by interlaminar air pockets (separated by 2.1 nm distances). The UV effect is also explained. UV light creates electron-hole pairs, with the holes reacting with lattice oxygen, creating surface oxygen vacancies, while the electrons reduce V5+to V3+. The oxygen vacancies are met by water, and it is this water absorbency by the vanadium surface that makes it hydrophilic. By extended storage in the dark, water is replaced by oxygen and hydrophilicity is once again lost.
Potential applications[edit]
Active recent research on superhydrophobic materials might eventually lead to industrial applications. For example, a simple routine of coating cotton fabric with silica[32] ortitania[33] particles by sol-gel technique has been reported, which protects the fabric from UV light and makes it superhydrophobic. Also, an efficient routine has been reported for making polyethylene superhydrophobic and thus self-cleaning.[34]—99% of dirt absorbed on such surface is easily washed away. Patterned superhydrophobic surfaces also have promise for lab-on-a-chip microfluidic devices and can drastically improve surface-based bioanalysis.[35]
Rabies
For other uses, see Rabies (disambiguation).
| Rabies | |
|---|---|

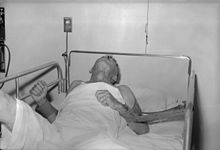
A dog with rabies in the paralytic (post-furious) stage
| |
| Classification and external resources | |
| Specialty | Infectious disease |
| ICD-10 | A82 |
| DiseasesDB | 11148 |
| MedlinePlus | 001334 |
| eMedicine | med/1374 eerg/493ped/1974 |
| Patient UK | Rabies |
| MeSH | D011818 |
| Orphanet | 770 |
Rabies is a viral disease that causes acute inflammation of the brain in humans and other warm-blooded animals.[1] Early symptoms can include fever and tingling at the site of exposure.[1] These symptoms are followed by one or more of the following symptoms: violent movements, uncontrolled excitement, fear of water, an inability to move parts of the body, confusion, and loss of consciousness.[1] Once symptoms appear, death nearly always results.[1] The time period between contracting the disease and the start of symptoms is usually one to three months; however, this time period can vary from less than one week to more than one year.[1] The time is dependent on the distance the virus must travel to reach the central nervous system.[2]
Rabies is caused by lyssaviruses including: rabies virus and Australian bat lyssavirus.[3] Rabies is spread when an infected animal scratches or bites another animal or human.[1] Saliva from an infected animal can also transmit rabies if the saliva comes into contact with the eyes, mouth, or nose.[1] Globally, dogs are the most common animal involved.[1] More than 99% of rabies cases in countries where dogs commonly have the disease are caused by dog bites.[4] In the Americas, bat bites are the most common source of rabies infections in humans, and less than 5% of cases are from dogs.[1][4] Rodents are very rarely infected with rabies.[4] The rabies virus travels to the brain by following the peripheral nerves. The disease can only be diagnosed after the start of symptoms.[1]
Animal control and vaccination programs have decreased the risk of rabies from dogs in a number of regions of the world.[1]Immunizing people before they are exposed is recommended for those who are at high risk. The high-risk group includes people who work with bats or who spend prolonged periods in areas of the world where rabies is common.[1] In people who have been exposed to rabies, the rabies vaccine and sometimes rabies immunoglobulin are effective in preventing the disease if the person receives the treatment before the start of rabies symptoms.[1] Washing bites and scratches for 15 minutes with soap and water,povidone iodine, or detergent may reduce the number of viral particles and may be somewhat effective at preventing transmission.[1][5] Only a few people have survived a rabies infection after showing symptoms, and this was with extensive treatment known as the Milwaukee protocol.[6]
Rabies causes about 26,000 to 55,000 deaths worldwide per year.[1][7] More than 95% of rabies deaths occur in Africa and Asia.[1]Rabies is present in more than 150 countries and on all continents but Antarctica.[1] More than 3 billion people live in regions of the world where rabies occurs.[1] A number of countries, including Australia, Canada, Japan, the United States, and Western Europe, do not have rabies among dogs.[8][9] Many small island nations do not have rabies at all.[10]
Contents
[hide]Signs and symptoms[edit]
The period between infection and the first flu-like symptoms is typically 2 to 12 weeks in humans. Incubation periods as short as four days and longer than six years have been documented, depending on the location and severity of the contaminated wound, and the amount of virus introduced. Signs and symptoms may soon expand to slight or partial paralysis, anxiety, insomnia, confusion, agitation, abnormal behavior, paranoia, terror, and hallucinations, progressing to delirium.[2][11] The person may also have hydrophobia.[citation needed]
Death mostly occurs 2 to 10 days after first symptoms. Survival is rare once symptoms have presented, even with the administration of proper and intensive care.[12] Jeanna Giese, who in 2004 was the first patient treated with the Milwaukee protocol,[13] became the first person ever recorded to have survived rabies without receiving successful post-exposure prophylaxis. An intention-to-treat analysis has since found this protocol has a survival rate of about 8%.[14]
Hydrophobia[edit]
Hydrophobia ("fear of water") is the historic name for rabies.[15] It refers to a set of symptoms in the later stages of an infection in which the person has difficulty swallowing, shows panic when presented with liquids to drink, and cannot quench his or her thirst. Any mammals infected with the virus may demonstrate hydrophobia.[16]
Saliva production is greatly increased, and attempts to drink, or even the intention or suggestion of drinking, may cause excruciatingly painful spasms of the muscles in the throat and larynx. This can be attributed to the fact that the virus multiplies and assimilates in thesalivary glands of the infected animal for the purpose of further transmission through biting. The infected animal's ability to transmit the virus would reduce significantly if it could swallow saliva and water.[17]
Hydrophobia is commonly associated with furious rabies, which affects 80% of the infected people. The remaining 20% may experience a paralytic form of rabies that is marked by muscle weakness, loss of sensation, and paralysis; this form of rabies does not usually cause fear of water.[16]
Cause[edit]
Rabies is caused by a number of lyssaviruses including: rabies virus and Australian bat lyssavirus.[18]
The rabies virus is the type species of the Lyssavirus genus, in the family Rhabdoviridae, order Mononegavirales. Lyssaviruses have helical symmetry, with a length of about 180 nm and a cross-section of about 75 nm.[19] These viruses are enveloped and have a single-stranded RNA genome with negative sense. The genetic information is packed as a ribonucleoprotein complex in which RNA is tightly bound by the viral nucleoprotein. The RNA genome of the virus encodes five genes whose order is highly conserved: nucleoprotein (N), phosphoprotein (P), matrix protein (M), glycoprotein (G), and the viral RNA polymerase (L).[20]
Once within a muscle or nerve cell, the virus undergoes replication. The trimeric spikes on the exterior of the membrane of the virus interact with a specific cell receptor, the most likely one being the acetylcholine receptor, acetyl. The cellular membrane pinches in a procession known as pinocytosis and allows entry of the virus into the cell by way of an endosome. The virus then uses the acidic environment, which is necessary, of that endosome and binds to its membrane simultaneously, releasing its five proteins and single strand RNA into the cytoplasm.[21]
The L protein then transcribes five mRNA strands and a positive strand of RNA all from the original negative strand RNA using free nucleotides in the cytoplasm. These five mRNA strands are then translated into their corresponding proteins (P, L, N, G and M proteins) at free ribosomes in the cytoplasm. Some proteins require post-translative modifications. For example, the G protein travels through the rough endoplasmic reticulum, where it undergoes further folding, and is then transported to the Golgi apparatus, where a sugar group is added to it (glycosylation).[21]
Where there are enough proteins, the viral polymerase will begin to synthesize new negative strands of RNA from the template of the positive strand RNA. These negative strands will then form complexes with the N, P, L and M proteins and then travel to the inner membrane of the cell, where a G protein has embedded itself in the membrane. The G protein then coils around the N-P-L-M complex of proteins taking some of the host cell membrane with it, which will form the new outer envelope of the virus particle. The virus then buds from the cell.[21]
From the point of entry, the virus is neurotropic, traveling quickly along the neural pathways into the central nervous system. The virus usually first infects muscle cells close to the site of infection, where they are able to replicate without being 'noticed' by the host's immune system. Once enough virus has been replicated, they begin to bind to acetyl choline receptors (p75NR) at the neuromuscular junction. [22] The virus then travels through the nerve cell axon via retrograde transport, as its P protein interacts with dynein, a protein present in the cytoplasm of nerve cells. Once the virus reaches the cell body it travels rapidly to the Central Nervous System (CNS), replicating in motor neurons and eventually reaching the brain. [2] After the brain is infected, the virus travels centrifugally to the peripheral and autonomic nervous systems, eventually migrating to the salivary glands, where it is ready to be transmitted to the next host.
Transmission[edit]
Main article: Rabies transmission
All warm-blooded species, including humans, may become infected with the rabies virus and develop symptoms. Birds were first artificially infected with rabies in 1884; however, infected birds are largely if not wholly asymptomatic, and recover.[23] Other bird species have been known to develop rabies antibodies, a sign of infection, after feeding on rabies-infected mammals.[24][25]
The virus has also been adapted to grow in cells of poikilothermic ("cold-blooded") vertebrates.[26][27] Most animals can be infected by the virus and can transmit the disease to humans. Infected bats,[28][29] monkeys, raccoons, foxes, skunks, cattle, wolves, coyotes, dogs, mongooses (normally yellow mongoose)[30] and cats present the greatest risk to humans.
Rabies may also spread through exposure to infected bears, domestic farm animals, groundhogs, weasels, and other wild carnivorans. Lagomorphs, such as hares and rabbits, and small rodents such as chipmunks, gerbils, guinea pigs, hamsters, mice, rats, and squirrels, are almost never found to be infected with rabies and are not known to transmit rabies to humans.[31] Bites from mice, rats, or squirrels rarely require rabies prevention because these rodents are typically killed by any encounter with a larger, rabid animal, and would, therefore, not be carriers.[32] The Virginia opossum is resistant but not immune to rabies.[33]
The virus is usually present in the nerves and saliva of a symptomatic rabid animal.[34][35] The route of infection is usually, but not always, by a bite. In many cases, the infected animal is exceptionally aggressive, may attack without provocation, and exhibits otherwise uncharacteristic behavior.[36] This is an example of a viral pathogen modifying the behavior of its host to facilitate its transmission to other hosts.
Transmission between humans is extremely rare. A few cases have been recorded through transplant surgery.[37] After a typical human infection by bite, the virus enters theperipheral nervous system. It then travels along the afferent nerves toward the central nervous system.[38] During this phase, the virus cannot be easily detected within the host, and vaccination may still confer cell-mediated immunity to prevent symptomatic rabies. When the virus reaches the brain, it rapidly causes encephalitis, the prodromal phase, and is the beginning of the symptoms. Once the patient becomes symptomatic, treatment is almost never effective and mortality is over 99%. Rabies may also inflame the spinal cord, producing transverse myelitis.[39][40]
Diagnosis[edit]
Rabies can be difficult to diagnose, because, in the early stages, it is easily confused with other diseases or with aggressiveness.[41] The reference method for diagnosing rabies is the fluorescent antibody test (FAT), an immunohistochemistry procedure, which is recommended by the World Health Organization (WHO).[42] The FAT relies on the ability of a detector molecule (usually fluorescein isothiocyanate) coupled with a rabies-specific antibody, forming a conjugate, to bind to and allow the visualisation of rabies antigen using fluorescent microscopy techniques. Microscopic analysis of samples is the only direct method that allows for the identification of rabies virus-specific antigen in a short time and at a reduced cost, irrespective of geographical origin and status of the host. It has to be regarded as the first step in diagnostic procedures for all laboratories. Autolysed samples can, however, reduce the sensitivity and specificity of the FAT.[43] The RT PCR assays proved to be a sensitive and specific tool for routine diagnostic purposes,[44] particularly in decomposed samples[45] or archival specimens.[46]The diagnosis can be reliably made from brain samples taken after death. The diagnosis can also be made from saliva, urine, and cerebrospinal fluid samples, but this is not as sensitive and reliable as brain samples.[43] Cerebral inclusion bodies called Negri bodies are 100% diagnostic for rabies infection but are found in only about 80% of cases.[19] If possible, the animal from which the bite was received should also be examined for rabies.[47]
The differential diagnosis in a case of suspected human rabies may initially include any cause of encephalitis, in particular infection with viruses such as herpesviruses,enteroviruses, and arboviruses such as West Nile virus. The most important viruses to rule out are herpes simplex virus type one, varicella zoster virus, and (less commonly) enteroviruses, including coxsackieviruses, echoviruses, polioviruses, and human enteroviruses 68 to 71.[48]
New causes of viral encephalitis are also possible, as was evidenced by the 1999 outbreak in Malaysia of 300 cases of encephalitis with a mortality rate of 40% caused by Nipah virus, a newly recognized paramyxovirus.[49] Likewise, well-known viruses may be introduced into new locales, as is illustrated by the recent outbreak of encephalitis due to West Nile virus in the eastern United States.[50] Epidemiologic factors, such as season, geographic location, and the patient's age, travel history, and possible exposure to bites, rodents, and ticks, may help direct the diagnosis.
Cheaper rabies diagnosis will become possible for low-income settings: accurate rabies diagnosis can be done at a tenth of the cost of traditional testing using basic light microscopy techniques.[51]
Prevention[edit]
Main article: Rabies vaccine
Almost all human cases of rabies were fatal until a vaccine was developed in 1885 by Louis Pasteur and Émile Roux. Their original vaccine was harvested from infected rabbits, from which the virus in the nerve tissue was weakened by allowing it to dry for five to ten days.[52] Similar nerve tissue-derived vaccines are still used in some countries, as they are much cheaper than modern cell culture vaccines.[53]
The human diploid cell rabies vaccine was started in 1967. Less expensive purified chicken embryo cell vaccine and purified vero cell rabies vaccine are now available.[47] Arecombinant vaccine called V-RG has been used in Belgium, France, Germany, and the United States to prevent outbreaks of rabies in undomesticated animals.[54] Immunization before exposure has been used in both human and nonhuman populations, where, as in many jurisdictions, domesticated animals are required to be vaccinated.[55]
The number of recorded human deaths from rabies in the United States has dropped from 100 or more annually in the early 20th century to one or two per year due to widespread vaccination of domestic dogs and cats and the development of human vaccines and immunoglobulin treatments. Most deaths now result from bat bites, which may go unnoticed by the victim and hence untreated.[56]
The Missouri Department of Health and Senior Services Communicable Disease Surveillance 2007 Annual Report states the following can help reduce the risk of contracting rabies:[57]
- Vaccinating dogs, cats, and ferrets against rabies
- Keeping pets under supervision
- Not handling wild animals or strays
- Contacting an animal control officer upon observing a wild animal or a stray, especially if the animal is acting strangely
- If bitten by an animal, washing the wound with soap and water for 10 to 15 minutes and contacting a healthcare provider to determine if post-exposure prophylaxis is required
September 28 is World Rabies Day, which promotes the information, prevention, and elimination of the disease.[58]
Treatment[edit]
Treatment after exposure can prevent the disease if administered promptly, generally within 10 days of infection.[19] Thoroughly washing the wound as soon as possible with soap and water for approximately five minutes is effective in reducing the number of viral particles.[59] Povidone-iodine or alcohol is then recommended to reduce the virus further.[60]
In the US, the Centers for Disease Control and Prevention recommends people receive one dose of human rabies immunoglobulin (HRIG) and four doses of rabies vaccine over a 14-day period.[61] The immunoglobulin dose should not exceed 20 units per kilogram body weight. HRIG is expensive and constitutes most of the cost of postexposure treatment, ranging as high as several thousand dollars. As much as possible of this dose should be injected around the bites, with the remainder being given by deep intramuscular injection at a site distant from the vaccination site.[21]
The first dose of rabies vaccine is given as soon as possible after exposure, with additional doses on days three, seven and 14 after the first. Patients who have previously received pre-exposure vaccination do not receive the immunoglobulin, only the postexposure vaccinations on days 0 and 3.[62]
The pain and side effects of modern cell-based vaccines are similar to flu shots. The old nerve-tissue-based vaccinations that require multiple painful injections into the abdomen with a large needle are inexpensive, but are being phased out and replaced by affordable World Health Organization intradermal-vaccination regimens.[47]
Intramuscular vaccination should be given into the deltoid, not gluteal area, which has been associated with vaccination failure due to injection into fat rather than muscle. In infants, the lateral thigh is recommended.[63]
Awakening to find a bat in the room, or finding a bat in the room of a previously unattended child or mentally disabled or intoxicated person, is regarded as an indication for post-exposure prophylaxis (PEP). The recommendation for the precautionary use of PEP in occult bat encounters where no contact is recognized has been questioned in the medical literature, based on a cost-benefit analysis.[64] However, a 2002 study has supported the protocol of precautionary administering of PEP where a child or mentally compromised individual has been alone with a bat, especially in sleep areas, where a bite or exposure may occur without the victim being aware.[65] Begun with little or no delay, PEP is 100% effective against rabies.[13] In the case in which there has been a significant delay in administering PEP, the treatment should be administered regardless, as it may still be effective.[21]
Induced coma[edit]
See also: Milwaukee protocol
In 2004, American teenager Jeanna Giese survived an infection of rabies unvaccinated. She was placed into an induced coma upon onset of symptoms and given ketamine,midazolam, ribavirin, and amantadine. Her doctors administered treatment based on the hypothesis that detrimental effects of rabies were caused by temporary dysfunctions in the brain and could be avoided by inducing a temporary partial halt in brain function that would protect the brain from damage while giving the immune system time to defeat the virus. After 31 days of isolation and 76 days of hospitalization, Giese was released from the hospital.[66] She survived with all higher level brain functions, but an inability to walk and balance.[67] On a podcast of NPR's Radiolab, Giese recounted, "I had to learn how to stand and then to walk, turn around, move my toes. I was really, after rabies, a new born baby who couldn't do anything. I had to relearn that all...mentally I knew how to do stuff but my body wouldn't cooperate with what I wanted it to do. It definitely took a toll on me psychologically. You know I'm still recovering. I'm not completely back. Stuff like balance and, um, I can't run normally."[68]
Giese's treatment regimen became known as the "Milwaukee protocol", which has since undergone revision with the second version omitting the use of ribavirin. Two of 25 patients survived when treated under the first protocol. A further 10 patients have been treated under the revised protocol, with a further two survivors.[14] The anesthetic drugketamine has shown the potential for rabies virus inhibition in rats,[69] and is used as part of the Milwaukee protocol.
On June 12, 2011, Precious Reynolds, an eight-year-old girl from Humboldt County, California, became the third reported person in the United States to have recovered from rabies without receiving PEP.[70]
Prognosis[edit]
In unvaccinated humans, rabies is almost always fatal after neurological symptoms have developed.[71]
Vaccination after exposure, PEP, is highly successful in preventing the disease if administered promptly, in general within 6 days of infection. Begun with little or no delay, PEP is 100% effective against rabies.[13] In the case of significant delay in administering PEP, the treatment still has a chance of success.[21]
5 of the first 43 patients (12%) treated with the Milwaukee protocol survived, and those receiving treatment survived longer than those not receiving the treatment.[72]
Epidemiology[edit]
Main article: Prevalence of rabies
In 2010, an estimated 26,000 people died from rabies, down from 54,000 in 1990.[7] The majority of the deaths occurred in Asia and Africa.[71] India has the highest rate of human rabies in the world, primarily because of stray dogs,[73] whose number has greatly increased since a 2001 law forbade the killing of dogs.[74] Effective control and treatment of rabies in India is also hindered by a form of mass hysteria known as puppy pregnancy syndrome (PPS). Dog bite victims with PPS (both male and female) become convinced that puppies are growing inside them, and often seek help from faith healers rather than from conventional medical services. In cases where the bite was from a rabid dog, this decision can prove fatal. Dr. Nitai Kishore Marik, former district medical officer of West Midnapur, states "I have seen scores of cases of rabies that reached our hospitals very late because of the intervention of faith healers. We could not save those lives."[75] An estimated 20,000 people die every year from rabies in India — more than a third of the global toll.[74] As of 2007, Vietnam had the second-highest rate, followed by Thailand; in these countries, the virus is primarily transmitted through canines (feral dogs and other wild canine species).[76] Another source of rabies in Asia is the pet boom. In 2006 China introduced population control for dogs in Beijing.[77]
The rabies virus survives in widespread, varied, rural fauna reservoirs. It is present in the animal populations of almost every country in the world except Australia and New Zealand.[78] Australian bat lyssavirus (ABLV), discovered in 1996, is similar to rabies and is believed to be prevalent in native bat populations.
In Asia and in parts of the Americas and Africa, dogs remain the principal host. Mandatory vaccination of animals is less effective in rural areas. Especially in developing countries, pets may not be privately kept and their destruction may be unacceptable. Oral vaccines can be safely distributed in baits, a practice that has successfully reduced rabies in rural areas of Canada, France, and the United States. In Montréal, Quebec, Canada, baits are successfully used on raccoons in the Mont-Royal Park area. Vaccination campaigns may be expensive, and cost-benefit analysis suggests baits may be a cost-effective method of control.[79] In Ontario, a dramatic drop in rabies was recorded when an aerial bait-vaccination campaign was launched.[80]
Rabies is common among wild animals in the US. Bats, raccoons, skunks and foxes account for almost all reported cases (98% in 2009). Rabid bats are found in all 48 contiguous states. Other reservoirs are more limited geographically; for example, the raccoon rabies virus variant is only found in a relatively narrow band along the East Coast. Due to a high public awareness of the virus, efforts at vaccination of domestic animals and curtailment of feral populations, and availability of postexposure prophylaxis, incidents of rabies in humans are very rare. A total of 49 cases of the disease was reported in the country between 1995 and 2011; of these, 11 are thought to have been acquired abroad. Almost all domestically acquired cases are attributed to bat bites.[81]
In Switzerland, the disease has been virtually eradicated after scientists placed chicken heads laced with live attenuated vaccine in the Swiss Alps.[80] The foxes of Switzerland, proven to be the main source of rabies in the country, ate the chicken heads and immunized themselves.[80]
History[edit]
Etymology[edit]
The term is derived from the Latin rabies, "madness".[82] This, in turn, may be related to the Sanskrit rabhas, "to do violence". The Greeks derived the word lyssa, from lud or "violent"; this root is used in the name of the genus of rabies Lyssavirus.[83]
Impact[edit]
Rabies has been known about since around 2000 B.C.[84] The first written record of rabies is in the Mesopotamian Codex of Eshnunna (circa 1930 BC), which dictates that the owner of a dog showing symptoms of rabies should take preventive measure against bites. If another person were bitten by a rabid dog and later died, the owner was heavily fined.[85]
Rabies appears to have originated in the Old World, the first epizootic in the New World occurring in Boston in 1768.[86] It spread from there, over the next few years, to various other states, as well as to the French West Indies, eventually becoming common all across North America.
Rabies was considered a scourge for its prevalence in the 19th century. In France and Belgium, where Saint Hubert was venerated, the "St Hubert's Key" was heated and applied to cauterize the wound. By an application of magical thinking, dogs were branded with the key in hopes of protecting them from rabies. The fear of rabies was almost irrational, due to the insignificant number of vectors (mostly rabid dogs) and the absence of any efficacious treatment. It was not uncommon for a person bitten by a dog but merely suspected of being rabid, to commit suicide or to be killed by others.[83] This gave Louis Pasteur ample opportunity to test postexposure treatments from 1885.[87] In ancient times, the attachment of the tongue (the lingual frenulum, a mucous membrane) was cut and removed as this is where rabies was thought to originate. This practice ceased with the discovery of the actual cause of rabies.[88]
In modern times, the fear of rabies has not diminished, and the disease and its symptoms, particularly agitation has served as an inspiration for several works of zombie or similarly-themed fiction, often portraying rabies as having mutated into a stronger virus which fills humans with murderous rage or uncurable illness, bringing about a devastating, widespread pandemic.[89]
Other animals[edit]
Main article: Rabies in animals
Rabies is infectious to mammals; three stages are recognized. The first stage is a one- to three-day period characterized by behavioral changes and is known as the prodromal stage. The second is the excitative stage, which lasts three to four days. This stage is often known as "furious rabies" for the tendency of the affected animal to be hyper-reactive to external stimuli and bite at anything near. The third is the paralytic stage and is caused by damage to motor neurons. Incoordination is seen, owing to rear limb paralysis, and drooling and difficulty swallowing is caused by paralysis of facial and throat muscles. Death is usually caused by respiratory arrest.[90]
Research[edit]
Rabies has the advantage over other pseudotyping methods for gene delivery in that the cell-targeting (tissue tropism) is more specific for difficult-to-reach sites, such as thecentral nervous system without invasive delivery methods, as well as being capable of retrograde tracing (i.e., going against the flow of information at synapses) in neuronal circuits.[91]
Recent evidence indicates artificially increasing the permeability of the blood–brain barrier, which normally does not allow most immune cells across, promotes viral clearance.[92][93]
List of disk magazines
This article contains a list of magazines distributed on cassette, floppy disk, CD-ROM, or DVD-ROM — collectively referred to as disk magazines (or diskmags).
Alphabetical list[edit]
0–9[edit]
A[edit]
- Adventurer (ZX Spectrum, 1995–2004, Russian/English [#14-#15 issues])
- El Afghano (IBM-PC)
- Alive (Atari ST/Atari Falcon)
- Amber (IBM-PC, 1998–1999)
- AMnews (Amiga, 1988–1989)
- AnotherMag (IBM-PC)
- Apple Talk (Apple)
- Autark (IBM-PC, 1996, English/German)
B[edit]
- Bad News (IBM-PC, 1994–1996, English/Polish)
- Bain (IBM-PC)
- Batsch (IBM-PC, 1999, German)
- Beam (IBM-PC, 1998–1999)
- Becanne (IBM-PC)
- Belgian Scene Report (IBM-PC)
- Big Blue Disk, ISSN 0893-2212 (IBM PC, 1986–1991; relaunched as On Disk Monthly (q.v.))
- Blackmail (IBM-PC, 1993–1996, German)
- Budyn (IBM-PC, 1996–2001, Polish/English)
C[edit]
- Caustic Verses (IBM-PC)
- CD Gold (Commodore CD32/CDTV, 1993), commercial release and first known CD-ROM based disk magazine for the Commodore Amiga; produced by Goldtech with editorial support from Infinite Frontiers[1]
- CD World (Commodore Amiga), titled dedidated to the Amiga CDTV, Amiga CD32 and Amiga CD-ROM systems; produced by Infinite Frontiers)
- Cee-64 Alive! (Commodore 64, relaunched as Commodore Cee (q.v.))
- Ceibe (IBM-PC, 1999–2000, Spain)
- Cheese (IBM-PC, 1996–1997)
- Chromasette (TRS-80 Color Computer)
- CLI (IBM-PC)
- CLOAD (TRS-80, 1978–?)
- Commodore Cee (all Commodore computers)
- Commodore Gazette (Commodore 64)
- Commodore Online Information Network (COIN!)
- Compute!'s Gazette (Commodore 64)
- Contrast (IBM-PC, 1994–1995)
- CooleR (IBM-PC)
- Cows and Snakefights (Commodore Amiga)
- Cream (IBM-PC)
- CURSOR (Commodore PET, 1978 to early 1980s)
- Cursor 64 (Commodore 64, early 1980s)
D[edit]
- Daskmig (IBM-PC)
- Death (IBM-PC)
- Defcon (IBM-PC)
- Demojournal (IBM-PC)
- DemoNews (IBM-PC)
- Digital Chat (IBM-PC)
- Digital Talk (Commodore 64)
- Disk (Apple II, 1983; business-oriented)
- Disk Busters Association (DBA) Diskmagazine (Atari ST/Falcon 030, 1991–1996)
- Disk Network (Apple II, c. 1983; geared to programmers)
- Disk User (BBC Micro, '80s)
- Diskazine (Apple II, 1982; geared to families)
- Diskworld (ISSN 0899-4838) (Apple Macintosh, 1988–1993; relaunched as Softdisk for Mac (q.v.))
- Domination (Commodore 64)
- Dragon (IBM-PC)
- Driven (Commodore 64, 1994–1995)
E[edit]
- European Top 20 (Commodore Amiga, 1992–1993)
- Evil (IBM-PC)
F[edit]
- Fanzine (Commodore Amiga, Spanish)
- Fatum (IBM-PC)
- The Final Frontier (Commodore Amiga) , first disk magazine dedicated solely to Star Trek; produced by Infinite Frontiers
- Flash (IBM-PC)
- Fleur (IBM-PC)
- Floppyland (IBM PC, 1990s)
- Fluxus (Apple Macintosh Hypercard-based)
- FutureView (Amstrad CPC)
G[edit]
- Gamer's Edge (IBM PC, 1990–1991)
- Gedan (Commodore Amiga, 1994–1995)
- Generation (Commodore Amiga)
- Genetic Dreams (Commodore 64, IBM-PC)
- Grapevine (Commodore Amiga, ?–1995)
- GURU (Commodore Amiga, ?–?)
H[edit]
- Hacker (IBM-PC, 1996–1999, Russian, Croatian)
- Harm (Hellraiser's alternative Russian magazine) (IBM-PC)
- Heroin (IBM-PC, 1998, English)
- Hoax (IBM-PC, 1992–1995, English)
- Hot-Mag (IBM-PC, 1994–1995, German)
- Hugi (IBM PC, 1996–present, English, German and Russian)
- Hugi.GER (IBM-PC, 2000–2005, German)
- HugiNews (IBM-PC, 1998–2000, English)
- Hydrophobia (IBM-PC, 1996–1997, Hungarian)
I[edit]
- I.B.Magazette (IBM PC, 1982–?)
- Image (IBM-PC)
- Imazine (Commodore Amiga)
- Imphobia (IBM PC)
- Incube (IBM-PC)
- Infinity (IBM-PC)
- Insomnia (Commodore Amiga)
J[edit]
K[edit]
L[edit]
- Lano (IBM-PC)
- Launch (Microsoft Windows and Mac OS 7.1 up, late 1990s - early 2000s)
- Legend (IBM-PC)
- Loadstar (ISSN 0886-4144) (Commodore 64, 1984–2010)
- Loadstar 128 (Commodore 128)
- Lookain Fanz (IBM-PC)
- Luna (IBM-PC)
- Lunchtime (Commodore Amiga and Acorn Archimedes, 1990–1996) (#1-Digital Dog Edition; #2 - Hamsters on the Prowl; #3 - Edward's Revenge; #4 - Yul Brynner's Memorial Toolshed; #5 - Wardrobe Racing for Foreigners; #6 - Danger: Unexploded Whippet)
M[edit]
- The Mag (IBM-PC)
- Maggie (Atari ST)
- Maniac Magazine (IBM-PC)
- Marriage Connection (IBM PC, 1989; computer-aided activities for married couples)
- M*A*R*S (IBM-PC)
- McDisk (Commodore Amiga)
- Megazin (Commodore Amiga)
- Mentor (IBM PC, c. 1983; mostly support programs for business software)
- MicroCode (IBM-PC)
- Microzine (Apple II, c. 1983; geared to pre-teens)
- Miggybyte (Commodore Amiga, 1995–1997)
N[edit]
O[edit]
- The Official Eurochart (Commodore Amiga)
- On Disk Monthly (IBM PC, 1991–1993; relaunched as Softdisk PC (q.v.))
- Ooze (IBM-PC)
P[edit]
- Pain (IBM PC)
- Parrot (IBM-PC)
- PC BusinessDisk (IBM PC, 1990–1991)
- PC Disk (IBM PC, c. 1983; mostly business)
- PC Disk Downunder (ISSN 1170-2737) (IBM-PC; Australia/New Zealand adaptation of Big Blue Disk)
- PC Life (IBM PC, 1988)
- Platinum (IBM-PC, German)
- Pornograffitti (Commodore 64, 1992-?, Canada)
- Pressure (Commodore Amiga)
- The Product (IBM-PC)
- Pulse (IBM-PC)
Q[edit]
R[edit]
S[edit]
- Satanic Rites (Commodore Amiga)
- Savage (IBM-PC)
- Savage Charts (IBM-PC)
- Saxonia (IBM-PC)
- The Scene Post (IBM-PC)
- Scene World Magazine (Commodore 64, 2000–present)
- scenedicate (Dreamcast, 2005–present)
- Scenial (IBM-PC)
- Schwugi (IBM-PC)
- Shine (IBM-PC)
- Showtime (Commodore Amiga)
- Sinner (IBM-PC)
- Skyline (IBM-PC)
- Slonecznik (IBM-PC)
- Smok (IBM-PC)
- Smurffi (IBM-PC)
- Sneaker (IBM-PC)
- Soap (IBM-PC)
- Softdisk (ISSN 0886-4152) (Apple II, 1981–1995)
- Softdisk for Mac (Apple Macintosh, 1993–1998)
- Softdisk for Windows (Microsoft Windows, 1994–1999)
- Softdisk G-S (Apple IIgs, 1989–?)
- Softdisk PC (IBM PC, 1993–1998)
- SoftSide (various platforms, early 1980s; disk/cassette companion to paper magazine)
- Speed (Commodore Amiga)
- Splash (IBM-PC)
- Static Line (IBM-PC)
- Stream CD-ROM Digizine (IBM-PC)
- Subkult (IBM-PC)
- Subliminal Extacy (ZX Spectrum)
- Suicide (IBM-PC, German)
- Sunray (IBM-PC)
- Syntax Error (IBM-PC)
T[edit]
- TAP.MAG (IBM-PC, 2000–2001, German)
- Terror News (IBM-PC, Hungarian)
- Testimony of the Ancients (IBM-PC)
- Total Disaster (IBM-PC)
- Totem (IBM-PC)
- Trashcan (Commodore Amiga, 1995–1999, Spanish, English)
- Trip! (IBM-PC)
- Trip 2 Hell (IBM-PC)
U[edit]
- Undercover Magascene (Atari ST) (merged with Alive Disk Magazine in 2000, but re-animated in 2001)
- Underground News (Commodore 64 1990-1994 - Canada)
- Upstream (Commodore Amiga)
- UpTime (various platforms, 1984–1990)
- El Usuario (IBM-PC; Latin American adaptation of Big Blue Disk)
V[edit]
- Vagina (IBM-PC)
- Vandalism (Commodore 64)
- Versus (IBM-PC)
- Vision (Commodore 64, 1993–1996)
- Vixel (Commodore VIC-20, early 1980s)
- The Voice (IBM-PC)
- v.O.L.V.o (IBM-PC)
W[edit]
- What (IBM-PC)
- WildMag (IBM-PC, 2000–2001, German)
- Window (Apple II, 1982; educational)
- Worldcharts (IBM-PC)
- Wrotki (IBM-PC)
X[edit]
Y[edit]
Z[edit]
- Zeitenwanderer (IBM-PC, German)
- ZINE (Amiga, IBM-PC from issue #12)
















No comments:
Post a Comment